South Western Sydney Local Health District (SWSLHD) has started a trial of an AI-powered wearable device to monitor COVID+ patients at home, joining Murrumbidgee LHD and the Howard Springs quarantine facility in Darwin in using the technology.
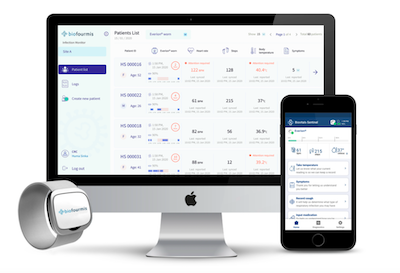
The remote monitoring system involves a wearable armband called Everion that monitors vital signs such as heart rate, respiratory rate, oxygen saturation, skin temperature and activity, with data sent to an app by Bluetooth and then onto a clinical dashboard for clinicians to monitor remotely.
The hardware was first developed by a Swiss company called Biovotion, which was bought last year by Boston-based start-up Biofourmis and integrated with that company’s Sentinel web-based dashboard software. It is being used by the Singapore Ministry of Health to monitor COVID-19-positive patients, and is distributed in Australia by Device Technologies.
It is also being used by Murrumbidgee LHD as part of a proof of concept study being run by Alcidion and eHealth NSW at Wagga Wagga Base Hospital to configure Alcidion’s Miya Memory mobile EMR to enable critical test results and risk indicators to be sent in real-time to mobile devices.
That project was expanded when the pandemic hit to allow data from the Biofourmis devices to be sent to Alcidion’s Miya Precision to create a dashboard for monitoring patients. Alcidion CEO Kate Quirke said the data is being combined with EMR data from the hospital’s Cerner eMR to give a complete picture for remote monitoring of patients in the home.
“Wagga Wagga Base doctors are also using the Miya Memory mobile app for access to the EMR data on their own devices,” Ms Quirke said.
Device Technologies’ senior business manager Jeff Reid said his team had been working at bringing out the technology for about three years.
“The hardware comes out of Switzerland but the software that accompanied it wasn’t ideal for clinical monitoring,” he said. “It was bit clunky, but then they partnered with a US based, AI-driven patient monitoring platform so they’ve now got a really nice, comprehensive clinical solution.
“We launched that in earnest probably late last year, and it was kind of timely given COVID. All of a sudden there was a significant demand for it.”
In the COVID projects the wearable is primarily monitoring heart rate, respiration rate, oxygen saturation, skin temperature and activity, but the device has other capabilities and Biofourmis plans to release a next-generation device that will have a blood pressure module as well, Mr Reid said.
In SWS LHD, nurses are monitoring patients from Campbelltown Hospital. The project is part of a study overseen by Professor Josephine Chow, head of the SWS LHD clinical innovation and business unit and is using the full Biofourmis package, including the Sentinel dashboard.
500 units have also been deployed to Howard Springs outside of Darwin, where the Northern Territory government is hosting a quarantine facility for Australians returning from overseas, who are being housed in converted dongas at the former mining camp.
“They contacted us about a week before all of these repatriated Australians were due to arrive,” Mr Reid said. “As of last week the system is up and running there and yielding some pretty valuable results by all accounts.”





Does this device require TGA approval?
It is classified as a Class IIa medical device