Australian developed mobile pain assessment app PainChek has entered the electronic medical record market through an integration using InterSystems’ IRIS for Health FHIR-based app development platform.
The partnership will see PainChek’s custom-built message formats converted to APIs using the HL7 and FHIR industry standards to allow it to integrate with hospital EMRs, including InterSystems’ TrakCare, as well as future FHIR-based home care systems.
It is currently integrated with a number of care management systems used in aged care and is contracted for use in more than 1300 aged care facilities in Australia, New Zealand, the UK and Singapore.
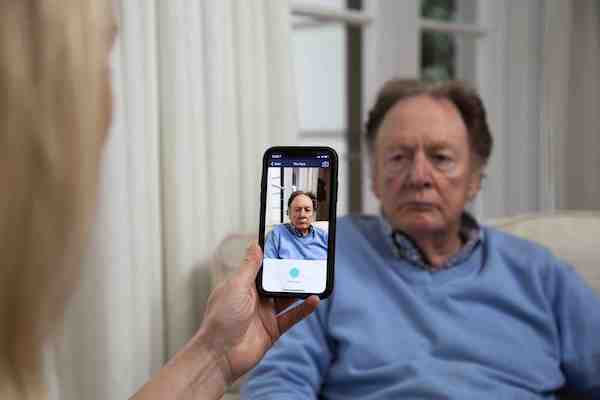
PainChek is thought to be the world’s first smartphone-based pain assessment and monitoring application and uses smart devices with cameras to accurately evaluate patients’ pain levels, particularly for people who can’t reliably describe their pain levels, are pre- or non-verbal, or are not lucid at the time.
It is offered as a software-as-a-service (SaaS) platform and allows pain assessments to be automatically entered into a patient’s record.
The agreement with InterSystems will be PainChek’s first move into hospitals, targeting use cases such as emergency departments, where triage would be more effective if accurate pain assessments could be performed on all patients.
It may also be useful for reducing readmissions or improving time to discharge, the two companies said. People with dementia occupy around 25 per cent of hospital beds and are more likely to exhibit delirium, which is associated with longer hospital stays.
PainChek CEO Philip Daffas said the partnership would accelerate the company’s entry into new markets like hospitals.
“We know from experience in aged care how important it is to connect to EMR systems,” Mr Daffas said. “InterSystems IRIS for Health makes that easy and scales up our capacity to connect into hospitals and, in the future, other sectors like home care that would also benefit from easy to use and reliable pain assessment.”
PainChek has regulatory clearance as a medical device in Australia, the UK, Europe, Singapore, Canada and New Zealand. An application to the US FDA is currently in progress.
Photo courtesy PainChek.